Groundwater contaminants
- Uranium
- Technetium-99
- Iodine-129
- Tritium
- Carbon tetrachloride
- Chromium
- Nitrates
- Strontium-90
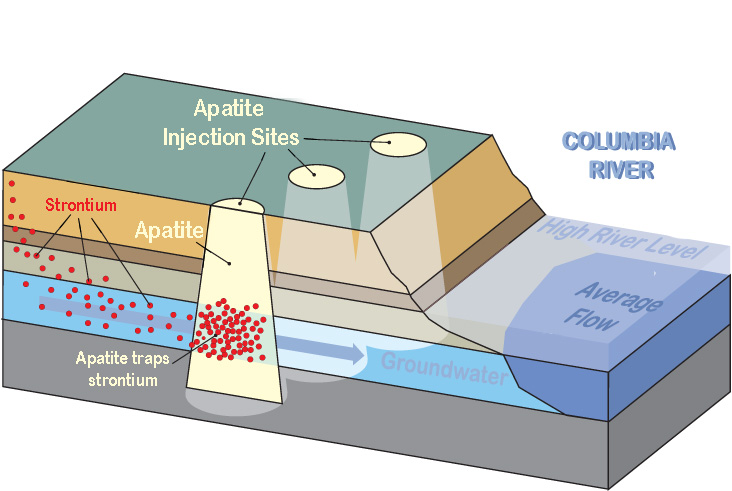
All radioactive contaminants have the potential to cause cancer. Chemical contaminants can cause cancer and other health effects. At Hanford's 100 Area, along the Columbia River, strontium-90 and hexavalent chromium have been a serious concern. In the past, these contaminants have entered the Columbia River at unacceptable levels.
As the diagram shows, when workers inject a phosphate mineral called "apatite" into the aquifer, it acts as a trap and separates the strontium-90 from the groundwater. This process of using apatite as a barrier has been used to prevent most of the strontium-90 from reaching the Columbia River. However, use of this method is limited and very expensive.
Radioactive Isotopes
Description
- Is produced as a by-product of fission and is a main constituent of nuclear waste and fallout
- Emits beta radiation.
- Half-life is 30 years.
- One of most common isotopes used in industry (moisture-density gauges, well-logging devices, thickness gauges, etc.).
Health concerns
- Exposure can be through ingestion, inhalation, or just being near it (since it emits gamma radiation).
- Behaves like potassium in the body and distributes throughout the body.
- In humans, is flushed from the body fairly quickly.
Exposure levels
- EPA has established a maximum contaminant level of 4 millirem per year for beta particle and photon radioactivity from man-made radionuclides in drinking water. Cesium-137 would be covered under this MCL. The average concentration of cesium-137 which is assumed to yield 4 mrem/year is 200 picocuries per liter. If other radionuclide's which emit beta particles and photon radioactivity are present, the sum of the annual dose from all the radionuclides must not exceed 4 mrem/year.
Description
- Is produced as a by-product of fission and is a main constituent of nuclear waste and fallout.
- Extremely weak beta emitter.
- Very long half-life: 15.7 million years.
- Water soluble, and moves very easily from atmosphere through the groundwater to living creatures.
Health concerns
- When ingested, most passes from the body, and about 30 percent goes to the thyroid.
- In humans, half of the remaining iodine leaves the body every 100 days.
- Long-term chronic doses can cause thyroid cancer.
- Can be absorbed by crops and end up in milk products.
Exposure levels
- Exposure to iodine-129, as to all radionuclides, results in increased risk of cancer.
- EPA has established a maximum contaminant level of 4 millirem (mrem) per year for beta particle and photon radioactivity from man-made radionuclides in drinking water. Iodine-129 would be covered under this MCL. The average concentration of iodine-129 which is assumed to yield 4 mrem/year is 1 picocurie per liter. If other radionuclides which emit beta particles and photon radioactivity are present, the sum of the annual dose from all the radionuclides must not exceed 4 mrem/year.
Description
- Is produced as a by-product of fission and is a main constituent of nuclear waste and fallout.
- Considered one of the more hazardous constituents of nuclear waste.
- Moderate beta emitter.
- Half-life is 29.1 years.
- Is relatively immobile.
- Biologically acts like calcium — lodges in bones, teeth, and bone marrow.
Health concerns
- In humans, can cause cancer in bones and blood-forming organs.
- Can cause anemia, abnormal bleeding, and inability to fight diseases.
Exposure levels
- Exposure to strontium-90, as to all radionuclides, results in increased risk of cancer. EPA has established a maximum contaminant level of 4 millirem (mrem) per year for beta particle and photon radioactivity from man-made radionuclides in drinking water. Strontium-90 would be covered under this MCL. The average concentration of strontium-90 which is assumed to yield 4 mrem/year is 8 picocuries per liter. If other radionuclides which emit beta particles and photon radioactivity are present, the sum of the annual dose from all the radionuclides must not exceed 4 mrem/year.
Description
- Is produced as a by-product of fission and is a main constituent of nuclear waste and fallout.
- Weak beta emitter.
- Long half-life of 212,000 years.
- Water soluble and highly mobile.
- There are currently limited methods to capture it in the environment.
- Can be absorbed by plants.
- Has no significant industrial use, though shorter-lived parent, Tc-99m, is the most widely-used radioactive isotope for medical diagnostic studies.
- Exposure is through ingestion of contaminated water or plants.
- In humans, concentrates in thyroid and gastrointestinal tract, but body excretes it quickly, half of it every 60 hours.
- Exposure to technetium-99, as to all radionuclides, results in increased risk of cancer. EPA has established a maximum contaminant level of 4 millirem (mrem) per year for beta particle and photon radioactivity from man-made radionuclides in drinking water. Technetium-99 would be covered under this MCL. The average concentration of technetium-99 which is assumed to yield 4 mrem/year is 900 picocuries per liter. If other radionuclides which emit beta particles and photon radioactivity are present, the sum of the annual dose from all the radionuclides must not exceed 4 mrem/year.
Description
- A radioactive form of hydrogen with two neutrons. (Nonradioactive hydrogen has none.)
- Weak beta emitter.
- Half-life is 12.3 years.
- Bonds to oxygen to create radioactive water molecules, called “tritiated” water.
- Highly mobile.
- Widely used in industry for illuminated signs and dials, biochemical research tracers, and more. Tritium gas is not readily taken up in the body and is not considered a health hazard.
Health concerns
- Exposure is by ingesting tritium as contaminated water and skin contact.
- In humans, is excreted through urine within a month or so.
Exposure levels
- Exposure to tritium, as to all radionuclides, results in increased risk of cancer. EPA has established a maximum contaminant level of 4 millirem (mrem) per year for beta particle and photon radioactivity from man-made radionuclides in drinking water. Tritium would be covered under this MCL. The average concentration of tritium which is assumed to yield 4 mrem/year is 20,000 picocuries per liter. If other radionuclides which emit beta particles and photon radioactivity are present, the sum of the annual dose from all the radionuclides must not exceed 4 mrem/year.
Description
- All uranium isotopes are radioactive.
- Uranium-238 is the most common naturally-occurring isotope of uranium.
- Alpha, beta, and weak gamma emitter.
- The most abundant isotopes have very long half-lives of up to 4.5 billion years.
- Has a long series of decay products, raising health concerns.
- Extremely dense and heavy metal.
Health concerns
- Chemically as well as radioactively toxic.
- Greatest health risk is from chemical toxicity to kidneys.
- Can accumulate in bones.
- Very immobile.
Exposure levels
- EPA maximum level for all uranium isotopes in drinking water is 30 μg/L (micrograms/liter) of drinking water.
Toxic Chemicals
Description
- Moves readily through soil.
- Colorless, clear, heavy liquid; sweet aromatic odor similar to chloroform.
- Carbon tetrachloride has a low potential to bioaccumulate.
- Evaporates quickly.
- Breaks down very slowly.
- Highly mobile.
- When it breaks down it forms compounds, such as chloroform, that destroy ozone in upper atmosphere.
- Was used for aerosol propellant, dry cleaning agents, rubber cement, refrigerator coolants, cleaning and degreasing fluids, and in fire extinguishers (now banned due to health risk).
- Not naturally found in the environment.
Health concerns
- Probably carcinogenic.
- Long-term exposure can cause damage to liver, kidney, and central nervous system.
- Acute exposure can cause liver, kidney, and lung damage when people are exposed to it in drinking water at levels above the EPA drinking water standards for relatively short periods of time.
Exposure levels
- EPA maximum level for drinking water is 5 micrograms per liter of carbon tetrachloride.
Description
- Chromium is a naturally occurring element found in rocks, animals, plants, soil, and in volcanic dust and gases.
- Chromium is odorless and tasteless.
- Chromium is present in the environment in several different forms — the most common are trivalent chromium (III), metallic (elemental) chromium (0), and hexavalent chromium (VI).
- Trivalent chromium (III) is an essential nutrient for humans.
- Trivalent chromium (III) has limited mobility in soils and water because it adsorbs to soil as a cation (example Cr3+) and precipitates out of solution at intermediate pHs in the environment.
- Hexavalent chromium (VI) is a known carcinogen by inhalation and is being studied to determine if it is a carcinogen by ingestion.
- Hexavalent chromium (VI) is a highly oxidized form of chromium with six fewer electrons than metallic chromium, and typically has an industrial origin.
- Hexavalent chromium (VI) is very mobile in the groundwater, and is the primary form of chromium that is remediated at the Hanford Site.
- Hexavalent chromium (VI) is present as complexes with oxygen that have negative charge (examples are chromate (CrO42-) and dichromate (Cr2O72-), and is generally mobile in soils and water.
- Hexavalent chromium (VI) is the substance investigated by Erin Brockovich in southern California, where it was used as a corrosion inhibitor by a power company as part of a natural gas pipeline system.
Health concerns
- Causes lung cancer in humans.
- Less carcinogenic if ingested, because stomach acids may convert it to nontoxic form.
- Direct eye contact with chromic acid or chromate dusts can cause permanent eye damage.
- Hexavalent chromium (VI) can irritate the nose, throat, and lungs. Repeated or prolonged exposure can damage the mucous membranes of the nasal passages and result in ulcers. In severe cases, exposure causes perforation of the septum (the wall separating the nasal passages).
- Short-term exposure can cause skin irritation or ulceration.
- Long-term exposure can cause damage to respiratory, gastrointestinal, immune, blood, skin, reproductive, ocular, liver, and kidney systems.
Exposure levels
- EPA maximum level for drinking water is 0.1 milligrams per liter for total chromium (the sum of all forms in water).
Description
- A complex of carbon triple-bonded to nitrogen, carrying a negative charge.
- Examples: hydrogen cyanide gas, sodium cyanide, potassium cyanide, and iron cyanides.
- Hydrogen cyanide is a colorless gas with a faint, almond-like odor.
- Cyanide salts have an almond-like odor when moist.
- Present in cigarette smoke.
- Fairly mobile.
- Used in electroplating, metallurgy, organic chemical and plastic production, photograph development, fumigation of ships, some mining processes, and metal extraction.
- Can be produced by certain bacteria, fungi, and algae.
- Found in combination with sulfur (such as thiocyanates) in some foods and plants (examples: almonds, millet sprouts, lima beans, soy, spinach, bamboo shoots, and cassava roots occurring naturally as part of sugars or other naturally-occurring compounds).
Health concerns
- Affects the nervous system (brain lesions, Parkinsonian-like symptoms, decreased verbal fluency, reduced information processing, coma), respiratory system (hyperventilation), cardiovascular system (shallow pulse, enlarged heart, and inaudible heart sounds), gastrointestinal system (nausea and vomiting), renal system (albinuria), and musculoskeletal system (generalized muscular rigidity).
- At fatal levels causes burning and dryness of throat, a warm sensation and a sense of suffocation, and may cause cardiovascular failure.
- Non-lethal exposures to hydrogen cyanide gas cause respiratory irritation, cough, altered sense of smell, nasal congestion, nose bleeds, coughing with blood, and shortness of breath in exposed workers.
- Other non-lethal effects include hypotension, heart palpitations, chest pains, nausea and vomiting, nervous system damage, and thyroid damage.
- Routes of exposure include inhalation, ingestion, and through skin contact.
Exposure levels
- EPA maximum level for drinking water is 200 micrograms per liter for total cyanide (the sum of all forms of cyanide in water).
Description
- Nitrates and nitrites are nitrogen-oxygen chemical units that combine with various compounds.
- Once taken into the body, nitrates are converted into nitrites.
- The greatest use of nitrates off of the Hanford Site is as a fertilizer.
- Chemically interchangeable with nitrites.
- Very soluble, does not bind to soils or evaporate, travels easily in groundwater.
Health concerns
- Interferes with blood’s ability to carry oxygen.
- Excessive levels of nitrate in drinking water have caused serious illness and sometimes death.
- In babies and children, exposure can cause shortness of breath and blueness of skin, when conversion of nitrates to nitrites interferes with oxygen-carrying capacity of child’s blood (can be acute, and health can deteriorate in a matter of days).
- Low blood-oxygen levels especially harmful to brain and heart.
- Long-term exposure can cause increased starchy deposits and hemorrhaging of the spleen.
Exposure levels
- EPA maximum level for drinking water for nitrates is 10 milligrams nitrate-nitrogen per liter and 1 milligram nitrate-nitrogen per liter for nitrites.
Description
- Used for a solvent.
- Very volatile.
- Production of trichloroethylene increased from just over 260,000 lbs in 1981 to 320 million lbs in 1991. Vapor degreasing of fabricated metal parts and some textiles accounts for 80 percent of its use.
- Clear, colorless or blue mobile liquid with sweet chloroform-like odor.
- Forms phosgene gas and hydrogen chloride, both toxic if inhaled.
Health concerns
- EPA has found trichloroethylene to potentially cause vomiting and abdominal pain from acute exposures at levels above the MCL.
- Trichloroethylene has the potential to cause liver damage from a lifetime exposure at levels above the MCL.
- Lodges in blood stream and fatty tissues.
- Toxicity affects many internal organs.
- There is some evidence that trichloroethylene may have the potential to cause cancer from a lifetime exposure at levels above the MCL.
Exposure levels
- EPA maximum level for drinking water is 5 micrograms per liter.
- The goal for drinking water for this contaminant is zero.