Managing pharmaceutical waste
Waste pharmaceuticals can affect the environment if they are not properly managed. Pharmaceuticals have been detected in water, sediments, and fish. We regulate businesses to ensure they properly manage waste pharmaceuticals.
As the agency charged with protecting Washington's environment, we also offer resources and technical assistance to help healthcare facilities comply with the law and protect the environment.

If you are an individual, go to Washington's Safe Medication Return Program to request free return envelopes or find a local drop-off site for household medications.
Learn more about pharmaceutical waste
Request our free poster
You can request our free How do I manage dangerous waste pharmaceuticals? flowchart to help you and your staff determine how to manage your dangerous waste pharmaceuticals.
Who must follow the special requirements?
The special requirements for the management of dangerous waste pharmaceuticals (WAC 173-303-555) apply to health care facilities that:
- Provide preventative, diagnostic, therapeutic, rehabilitative, or palliative care to improve the physical or mental condition of humans and animals,
OR - Sell or distribute pharmaceuticals.
To determine how to manage your dangerous waste pharmaceuticals, you must count BOTH your:
- Pharmaceutical dangerous waste, AND
- Nonpharmaceutical dangerous waste each month.
Unsure what types of waste you have? See our designation page.
Step 2: Check your waste count against monthly waste limits
| Monthly waste limits (QEL) |
|---|
| 2.2 pounds of dangerous waste with a quantity exclusion limit (QEL) of 2.2 pounds |
| 220 pounds of dangerous waste with a QEL of 220 pounds |
| 220 pounds of residue or contaminated soil, water, or other debris resulting from the cleanup of a spill to land or water of any dangerous waste with a QEL of 2.2 pounds. |
Step 3: Determine your management options
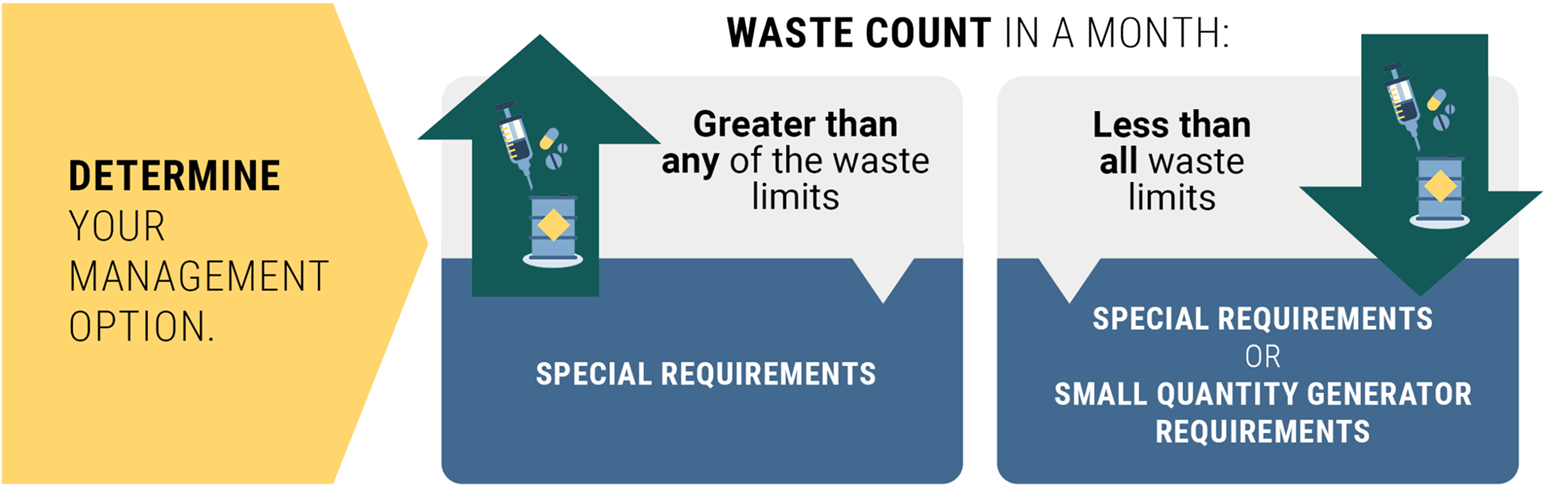
Compare your waste count against the monthly waste limits to now determine your management option(s).
If your waste count in a month is:
- Greater than ANY of the waste thresholds, follow the special requirements.
- Less than ALL waste thresholds, you can choose to follow either:
- Special requirements, or
- Standard dangerous waste requirements.
Management options
Special requirements
If you determine through the steps above that you must manage your dangerous waste pharmaceuticals using the special requirements, follow the steps below.
Need more help?
See our webinars below for more help understanding the new pharmaceutical rules or visit our Frequently asked questions page for more answers.
Guidance for hospitals and larger health care facilities
Watch the webinar our health care team conducted explaining how the new pharmaceutical rules apply to hospitals and larger health care facilities.
Available formats: YouTube | PDF slides
Guidance for clinics and smaller health care facilities
Watch the webinar our health care team conducted explaining how the new pharmaceutical rules apply to clinics and smaller health care facilities.
Available formats: YouTube | PDF slides
Related links
Contact information
Need help?
Contact our health care team